RLYB116
Complement Dysregulation
The complement system plays a central role in innate immunity, as well as in shaping adaptive immune responses. Dysregulation of the complement pathway has been linked to a growing number of diseases (see below), reinforcing its importance as a target for therapeutic intervention.
C5: A Proven Target in Complement Dysregulation
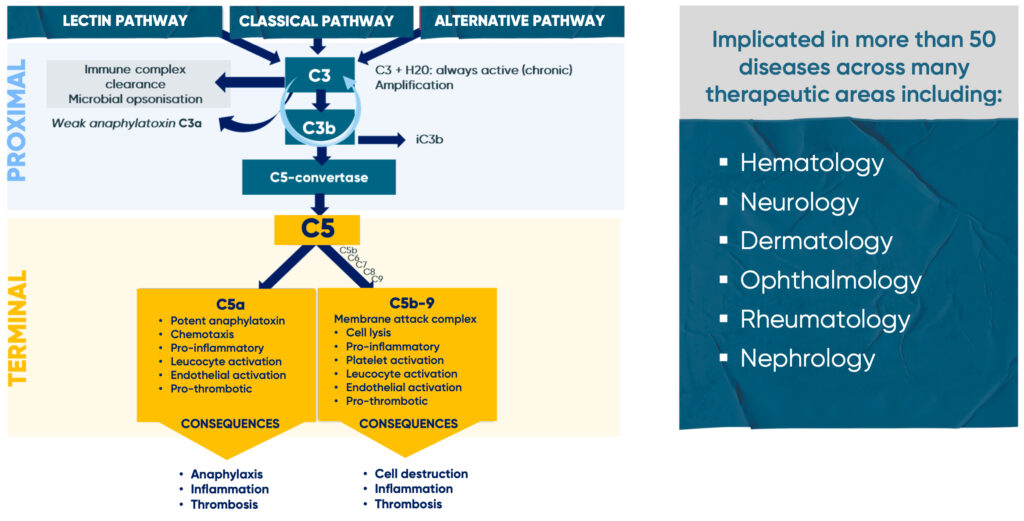
Antibody-based inhibitors of complement component 5 (C5) have been successfully developed to treat diseases that include paroxysmal nocturnal hemoglobinuria (PNH), refractory generalized myasthenia gravis (gMG), atypical hemolytic uremic syndrome (aHUS), and relapsing neuromyelitis optica spectrum disorder (NMOSD).
Prior to the formation of Rallybio, team members built a successful track record around the world in the design, development, and approval of C5 inhibitors to treat severe and rare diseases. The approval and use of antibody-based C5 inhibitors for patients demonstrates their value, and we believe that there is an opportunity to bring additional safe, effective, patient-friendly, and accessible C5 therapies to patients.
RLYB116
RLYB116 is a novel antibody mimetic fusion protein that includes an albumin-binding domain. RLYB116 has the potential to be a best-in-class, high-potency, long-acting C5 inhibitor that can be self-delivered by an autoinjector.
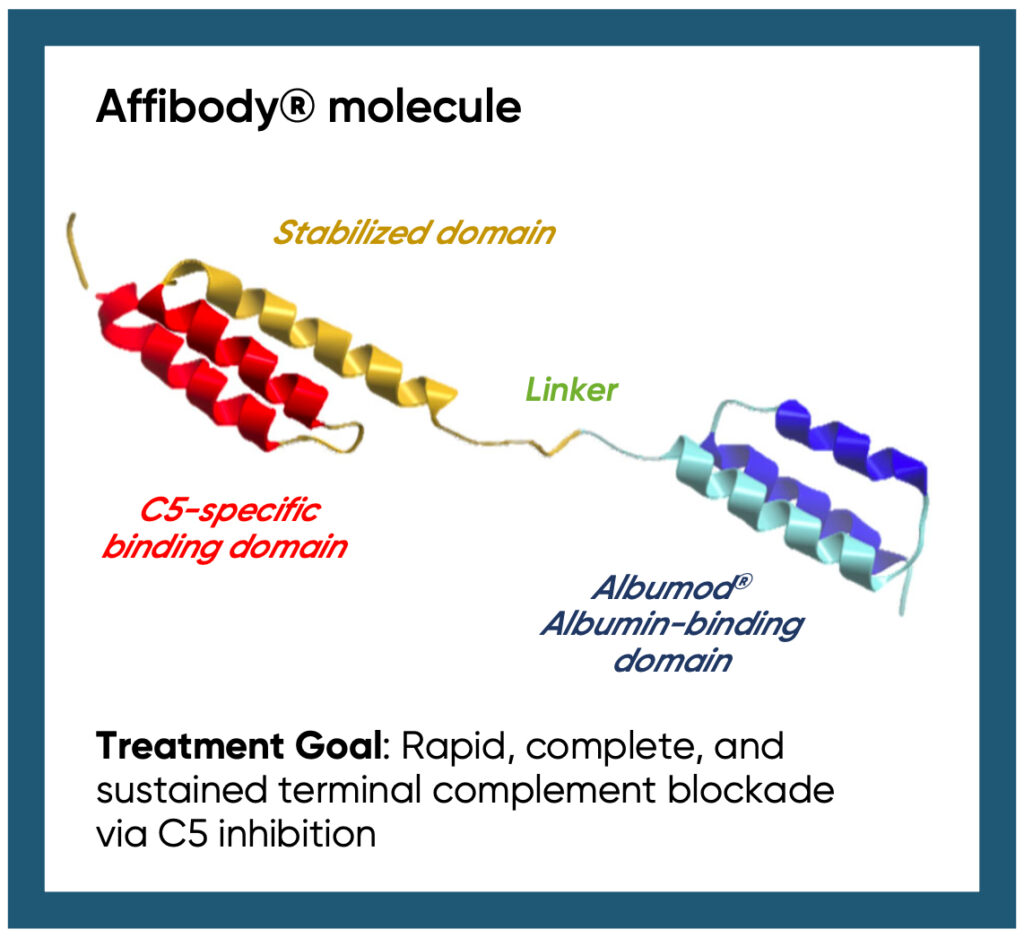
A novel fusion protein, RLYB116 consists of two, linked components:
- An Affibody® molecule with high affinity for C5, thereby inhibiting terminal complement activation, and
- Albumod™ technology that enables binding to serum albumin for the potential of an extended plasma half-life and broad tissue distribution
Because of its small compound size, RLYB116 may be subcutaneously administered in a small volume by an autoinjector. Furthermore, the potential for sustained, complete C5 inhibition and a long half-life in serum would likely lead to dosing no more than once a week.
RLYB116 Clinical Stage of Development
Phase 1 single and multiple ascending dose trial in healthy volunteers (Australia)
Potential Indications
C5 blockade to treat rare diseases in which complement dysregulation is a central or major factor; first indication to be disclosed upon completion of the ongoing Phase 1 program
Disease Mechanism
Over-activation of C5 is associated with excessive inflammation, tissue damage, and autoimmunity seen in diverse disease states.
Therapeutic Modality
Novel antibody mimetic (fusion protein consisting of two, linked components, Affibody® molecule and Albumod™ domain)
Mechanism of Action
Designed to provide rapid, complete, and sustained inhibition of C5 via subcutaneous injection
Mode of Administration
Self-delivery by autoinjector
Clinical Program Status
In November 2022, Rallybio announced first-in-human clinical data from the 100 mg dose cohort (N=8) in the single ascending dose portion of its Phase 1 trial of RLYB116 in healthy participants.
According to the preliminary results, all participants (N=6) receiving subcutaneous RLYB116 demonstrated a reduction in free C5 greater than 99% within 24 hours of dosing. The data suggest the potential for rapid and complete functional blockade of terminal complement activity. Additionally, terminal elimination half-life of RLYB116 was greater than 300 hours, supporting the potential for once-a-week or less frequent dosing.
In January 2023, the Company announced that it initiated the first cohort in the multiple ascending dose portion of the Phase 1 study during the fourth quarter of 2022.
